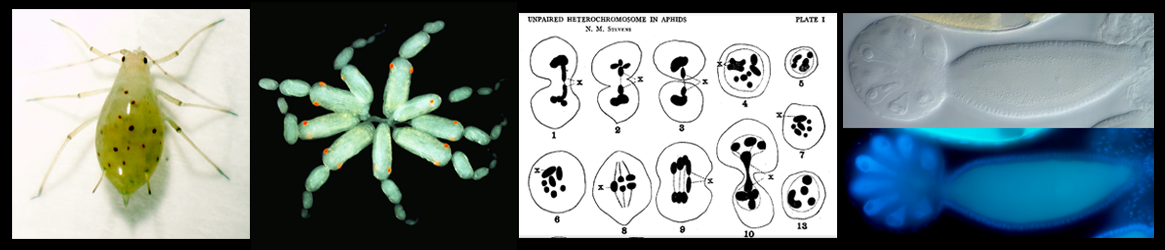
Polyphenism is a form of adaptive developmental plasticity in which the production of discrete, alternate phenotypes are cued by the environment. They present incredible opportunities to investigate how divergent developmental trajectories are directed by a single genome. Aphids—small, hemipteran insects that live on a diet of plant sap—are famous for their polyphenisms. In the case of the reproductive polyphenism, differences in day length determine whether mothers produce daughters that reproduce sexually, by laying fertilized eggs (oviparous sexual reproduction), or asexually, by allowing oocytes to complete embryogenesis within the mother without fertilization (viviparous parthenogenesis).
Parallel time lapse videos of a sexual and asexual female pea aphid laying an egg and giving birth, respectively. The egg-laying took 6.5 min, while the birth took almost an hour. Licensed under CC BY-SA 4.0.
Here is also a great video on the PBS platform from KQED and the NSF that describes a host of cool aphid phenomena, including teloscoping, the wing polyphenism, the ant symbiosis and parasitoidism.
Currently, my students and I are pursuing three areas of research into aphids, with a focus on the reproductive polyphenism.
Differences in patterning between oviparous and viviparous development
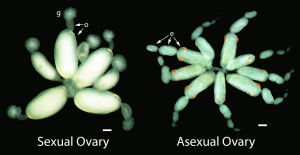
Oviparous and viviparous ovaries of sexual and asexual females, respectively. g, germarium; o, oocyte; e, embryo. Scale bars are both 100 μm.
Developing into a multicellular organism is a complex process, one that involves asymmetric activation of particular genes or asymmetric distribution of their products in the cells that support the developing oocyte and constitute the early embryo. These early assymmetries establish the primary axes of the embryo and positionally specify the fates of groups of cells.
Because sexual aphids lay eggs on leaves while asexual aphids give live birth, their ovaries look very different and the daughter eggs and embryos of these aphids develop very differently (amount of yolk, number and size of cells, timing of events, etc.) (Davis, 2012).
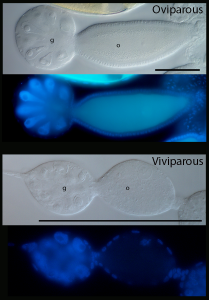
Above: Oviparous germarium and previtellogenic oocyte under bright field and DAPI. Below: Viviparous germarium and previtellogenic diploid oocyte, about 1/6th size of oviparous oocyte. g, germarium; o, previtellogenic oocyte. Scale bars are both 100 μm.
This phenomenon represents an extreme case of developmental plasticity in which development is altered due to changes in the environment with no change in the genome. Do these differences require changes in the mechanisms of early development such as axis specification and patterning? Inspired by this question, my students, my collaborators and I have worked to detect differences in the expression of genes that may play key roles in the early development of the progeny of sexual versus asexual pea aphids, focusing on homologs of the so-called “terminal patterning system” known from Drosophila (Bickel et al., 2013). In the future we plan to continue to characterize the extent of such differences, deepening our understanding of how such different developmental modes can be directed by the same genome.
Induction of reproductive fate

An asexual female pea aphid. Note the eyes of mature embryos within the ovaries that are visible through the cuticle.
A largely unanswered question concerns the nature of the developmental mechanisms that, in cases of developmental plasticity, control the “decision” to develop as one form versus another. In the case of aphid reproductive polyphenism, my students and I are investigating the nature of the molecular switch that specifies sexual versus asexual fate during embryonic development, discriminating among competing hypotheses for the role that juvenile hormone plays in the process and attempting to identify genes that are differentially expressed during early specification and subsequent differentiation.
The role of aphids in the history of genetics and embryology
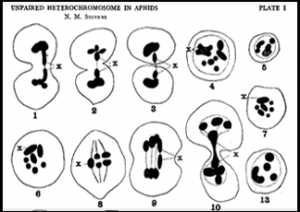
A plate from a 1909 paper by Nettie Stevens on aphid cytogenetics.
My students and I are also investigating the early 20th century work of cytogeneticist Nettie Stevens (co-discoverer, along with EB Wilson, of chromosomal sex determination) and her mentor TH Morgan on sex determination in aphids, which was conducted at both Bryn Mawr College and Columbia University (after Morgan moved there in 1904). We are working to make a case that, for both Stevens and Morgan, aphids played a key role in their ultimate adoption of the Mendelian chromosomal theory of inheritance.